Research
AVX001 is a highly potent and selective small molecule that blocks the activity of the key enzyme cPLA2α which is a key biomarker for poor prognosis in cancer. When blocking the cPLA2α enzyme activity with AVX001 the levels of arachidonic acid are reduced following regress of the tumor growth. Several key hallmarks of cancer development are affected, including programmed cell death, anti-tumor inflammation, inhibition of angiogenesis, and anti-tumor proliferation.
High cPLA2α levels correlate with metastasis and hence poor prognosis of several cancers. Even poor treatment outcomes of existing treatments have been shown to be correlated with high cPLA2α levels, and inhibiting cPLA2α in combination with existing standard of care treatments, for example radiation, increases the treatment response in preclinical models.
• Clinically validated drug candidate
• Well characterized mode-of-action
• Strong evidence for treating inflammatory driven cancers
• Skin friendly with excellent safety profile and limited skin reaction
• Cosmetically attractive gel formulation with high delivery of the drug candidate into the skin
Promising results in completed clinical trials
Clinical safety and efficacy studies of AVX001 have been completed in four phase 1 or 2 clinical studies. Two studies with a topical ointment formulation for the treatment of mild-to-moderate psoriasis, and two in a gel formulation for the treatment of atopic dermatitis and AK. In the latter, we conducted the Copenhagen Actinic Keratosis Study (COAKS), a 12-week single-center, randomized, vehicle-controlled, double-blind, hybrid clinical trial in adults with multiple AK lesions Olsen grade 1 or 2. In this study the AVX001 topical gel-formulation was found to be safe and tolerable, and effectiveness was observed for the highest dose. From the three other studies AVX001 was also found to be safe and effectiveness was shown in treating psoriasis patients.
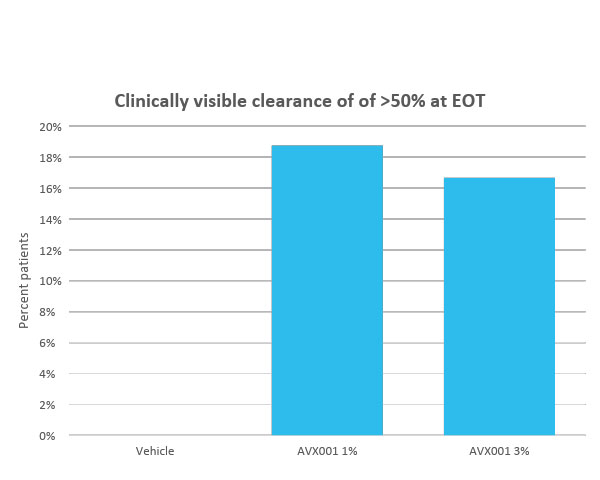
AVX001 3% reduced the number of AK lesions

AVX001 showed overall effectiveness
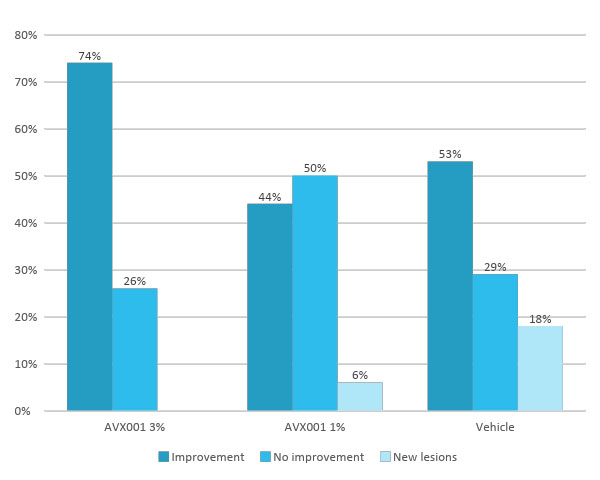
AVX001 acts more effectively in more severe AK-grade-2
BCC cell proliferation is highly dependent on the so-called Hedgehog signaling pathway, and the marketed drug vismodegib has been developed to block this pathway. AVX001 has been investigated in human BCC cell line model. The experiments demonstrated a potent dose dependent effect, being ten times more potent as compared to vismodegib. These data form a strong rationale for the planned clinical study in superficial basal cell carcinoma.